R.W. St. Germain
Dakota Technologies, Inc., Fargo, ND, USA
M.D. Einarson & A. Fure
Haley & Aldrich, Oakland, CA & Indianapolis, IN, USA
S. Chapman & B. Parker
University of Guelph, Guelph, ON, Canada
This article was originally published in the CPT'14 Proceedings
ABSTRACT: Early chlorinated DNAPL investigators adapted a visual dye staining technique to detect chlorinated DNAPLs in field samples in a quest to identify “source term” DNAPL responsible for dissolved phase plumes. The technique involves the addition of a small quantity of black powdered dye and water to soil sample jars. If present, the chlorinated solvents rapidly solvate the dye converting the dye’s appearance from black to a bright red, thus staining the chlorinated solvents and aiding investigators’ ability to detect any existing DNAPL. A laser-induced fluorescence (LIF) system which employs a similar dye-staining approach utilizing fluorescent changes in the dye (rather than chromatic) in the presence of NAPL has been designed, built and recently field-tested. The DyeLIF technique combines mature LIF technology with delivery of a dye ahead of the LIF sensor. The result is a continuously-advanced chlorinated solvent NAPL sensing system compatible with CPT and other direct push systems.
1. INTRODUCTION
Chlorinated solvents are a common organic contaminant class responsible for many of the largest groundwater contamination sites. The sources of these contaminants are often historical releases of dense non-aqueous phase liquids (DNAPLs) during storage or use of chlorinated solvents for metals degreasing, dry cleaning, and other uses (Pankow and Cherry 1996). These contaminants are denser than water and once spilled, the solvent often cascades down the soil profile driven by gravity, moving laterally along soil interfaces with lower permeability, eventually creating a complex waterfall-like structure of “ganglia” and “pools”. Below the water table, DNAPL constantly releases contamination by dissolution, so locating and remediating these hard to locate “source term” remnant globules of DNAPL is key to efficient and cost-effective remediation.
Direct push compatible laser-induced fluorescence (LIF) tools are currently available for real-time, high-resolution mapping of petroleum hydrocarbon and coal tar based NAPL source zones due to polycyclic aromatic hydrocarbon (PAH) content of these contaminants (Aldstadt et al. 2002). Unfortunately these LIF tools do not work with chlorinated solvent DNAPLs because chlorinated solvent molecules lack the aromatic structure required for efficient LIF response. While direct push tools exist for chlorinated solvents (USEPA 2004, 2005) they respond to both the aqueous and non-aqueous phases and struggle to differentiate the NAPL from high dissolved phase concentrations (McAndrews et al. 2003).
The most effective technique for specifically targeting and characterizing the DNAPL phase is to employ high-resolution vertical sampling where soil cores are obtained and carefully sampled and analyzed at small scales to try and locate any “needle in the haystack” ganglia (e.g. Parker et al. 2003). Small plugs of soil are removed from the cores at discrete horizons using a soil subsampler and extruded into a vial containing a small amount of Sudan IV or Oil Red O (ORO) hydrophobic dye are added to the soil along with water to create slurry. Any DNAPL in the soil rapidly solvates the ORO dye, converting it from a black powder to a bright red color, making DNAPL readily identifiable (Cohen et al. 1992; Parker et al. 2003). In this way the true source term at the site is located; giving engineers the information they need to determine how to best remediate the site. Unfortunately, sampling and testing with ORO is greatly dependent on good core recovery and quality, is labor intensive and significant hazardous waste is generated.
The authors recently demonstrated a direct push delivered sensor that is capable of accomplishing the ORO-like DNAPL-specific screening capability “on the fly” and in-situ. The new tool, the dye-enhanced laser induced fluorescence system or DyeLIF, is based on combining time-resolved LIF technology with the injection of a fluorescent dye ahead of the window to render non-fluorescent NAPLs fluorescent in-situ. It has potential to rapidly delineate DNAPL source zones in 3-D at sites where direct push is feasible, significantly improving prospects for targeted remediation.
2. TECHNOLOGY DESCRIPTION
Laser induced fluorescence (LIF) technologies such as the Ultra-Violet Optical Screening Tool (UVOST®) and the tar-specific green optical screening tool (TarGOST®) are popular tools for realtime, high-resolution mapping of petroleum hydrocarbons, creosotes, and coal tar based non-aqueous phase liquids (NAPLs). They are delivered using cone penetrometer test (CPT) and percussion direct push systems. They consist of a light source (laser), fiber optics strung through the rod string, optical detection and processing equipment. As the probe rods are advanced into the subsurface, pulses of excitation light are emitted from a sapphire window present near the base of the probe tip (or behind the tip and sleeve in the case of CPT). The emitted light, which is otherwise reflected (or scattered) by soil, is absorbed by any PAHs found in petroleum hydrocarbon and coal tar based NAPLs. These excited state PAHs quickly yield fluorescence which is transmitted back up to the ground surface via an optical fiber, where it is analyzed in real-time using data processing equipment located at the surface.
A schematic of the downhole function is shown in Figure 1. The probe functions by injecting an aqueous delivery fluid containing a proprietary hydrophobic dye through a small injection port that is situated 22 cm below the LIF window. As the probe is advanced through the subsurface the injected dye contacts the soil and quickly partitions into any DNAPL (if present). Standard LIF tooling is used to detect the dye-labeled chlorinated solvent DNAPLs. The dye is injected at a modest 1 mL/sec target rate in a controlled manner in order to lay down a “snails trail” of indicator dye along the side of the probe. Low dye injection rates avoid the risk of flushing DNAPL ganglia back into the soil grains and therefore outside LIF’s zone of optical interrogation. Automated recording of the flow rate of the dye solution and injection back-pressure yields useful information about the hydraulic conductivity, in a fashion similar to other direct push hydraulic profiling tools, such as the Geoprobe HPT™ (Hydraulic Profiling Tool) and Waterloo APS™ (Advanced Profiling System).
Testing to date has been conducted with percussion-advanced direct push systems. Hand-controlled push rates of 1.0 cm/sec (below the standard CPT minimum of 2 cm/sec) have been used to maximize the chance of small ganglia detection because the DyeLIF system acquires data at a fixed rate. At 1.0 cm/sec advance rate the average data spacing is 0.5 cm. We do not expect a significant change of performance at higher speeds other than an equivalent increase in data spacing and an accompanying “averaging out” of small but potentially important ganglia. At the time of this paper the system has not been deployed using CPT, but the hardware with the same basic design of current optical screening tool (OST) subs has been built. A trial with CPT advancement is expected to have occurred by the time the CPT14 conference takes place.
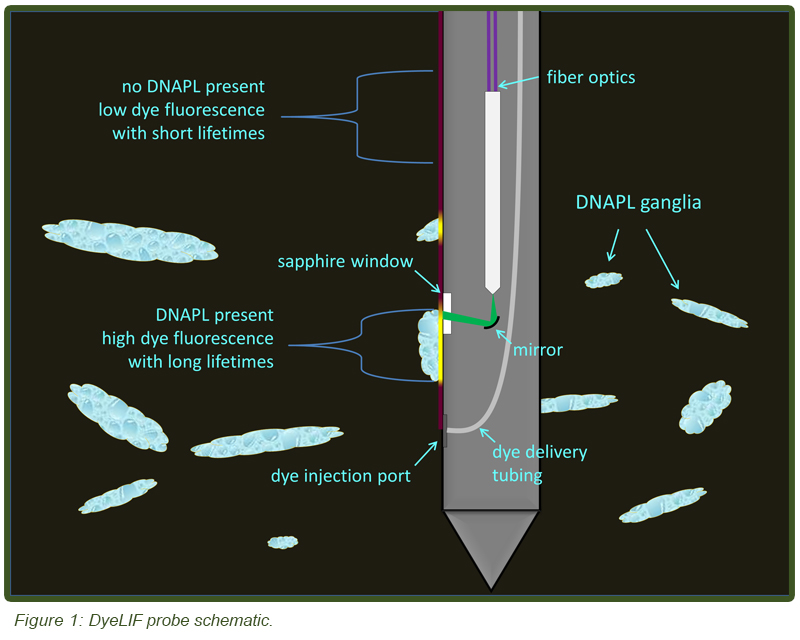

With DyeLIF virtually any NAPL can be detected, regardless of whether or not it contains innate fluorophores as is the case with petroleum, coal tar, and creosote. It is anticipated that the new LIF tool will also be very useful for detecting other PAH-starved petroleum hydrocarbons such as aviation gasoline and NAPLs of mono-aromatic compounds like benzene, toluene, ethyl-benzene, and xylenes (BTEX) that cannot be detected with conventional LIF technologies. Through extensive laboratory tests, a dye was identified that fluoresces well when the dye is solvated in common chlorinated solvents (e.g. trichloroethylene), but is otherwise a poor fluorophore when in particle form. This dye also undergoes a dramatic increase in fluorescence lifetime (decay time required for all the excited state dye molecules to return to the ground state after excitation) when solvated in ganglia. The dye is also relatively non-toxic (Rat LD50 (intraperitoneal) 4170 mg/kg) and not a known or suspected carcinogen. Extensive analytical testing on water left in contact with the dye for several days yielded no detectable levels of any listed VOCs or semi-VOCs, so the dye itself should not face excessive regulatory hurdles. The current injection rate of 1mL/sec results in approximately 0.11 g of dye injected with each meter of penetration (assuming 1 cm/sec advancement rate).
3. FIELD TESTING
3.1 Test Plan
The capability of the DyeLIF was recently demonstrated at a small footprint chlorinated spill site in Massachusetts. Tests of chlorinated solvent DNAPL recovered from the site revealed a mixture of 59% trichloroethylene, 34% 1,1,1-trichloroethane with various solvents and petroleum hydrocarbons making up the balance. Local geology in the demonstration area can be generally classified as fine sand and silt with intermittent silt and clay layers. A continuous silt layer is observed at 14 m below ground surface (bgs), which is consistent with other areas of the larger site. Previous investigations show the DNAPL impacted area is rather small, heterogeneously occupying an approximately 30 m x 30 m area, with DNAPL penetrating down to bedrock at about 27 m bgs. Figure 2 illustrates the limited footprint of the source term DNAPL.

The purpose of this demonstration was to validate the field performance of DyeLIF’s DNAPL sensing abilities in an exhaustive fashion. The two-week demonstration was designed to assess both the reliability of the tooling in normal production and to determine the sensing capabilities of the probe including limits of detection (LOD), susceptibility to false positives, and NAPL-specific responsivity. Our team developed the testing approach in order to account for (and attempt to control) the influence of extreme DNAPL distribution heterogeneity within a reasonable scope and budget:
- Week 1: Probe with DyeLIF in “full production mode” in an attempt to fully determine the architecture or nature and extent of the DNAPL distribution.
- Fully bound the DNAPL source zone, if possible.
- Achieve a number of co-located duplicate borings to assess small scale heterogeneity.
- Achieve “even coverage” of locations within the DNAPL affected zone.
- Week 2: Use the DyeLIF data generated in week one to determine optimal locations for confirmatory testing, involving collection of continuous cores and application of high resolution core subsampling methods, with a focus on (a) comparison of the “in-situ” DNAPL distribution inferred from DyeLIF probing with that from core subsampling applying both screening techniques and quantitative analyses of the soil subsamples, and (b) obtaining a wide range of DNAPL pore saturation soils for uphole analysis, ranging from clean to the most highly DNAPL saturated soil zones at the site.
- Photograph open cores, immediately cover in foil to avoid loss of VOCs, and sub-sample at high resolution (2-10 cm spacing) with four small sub-samples collected at each depth horizon using a plunger tool and placed in small sample vials (three 20 mL VOA vials, one containing Oil Red O and water for visual screening, one for DyeLIF screening, and one for moisture content and organic carbon analyses; and one in a 40 mL VOA containing methanol for quantitative analyses).
- Subject the four sub-samples to different tests including Oil Red O visual screening for DNAPL, bench-top DyeLIF, and analytical laboratory. The visual screening and quantitative analyses provide a means to compare DyeLIF with traditional methods (Griffin & Watson, 2002).
- Immediately after subsampling at each depth, scan with handheld photo ionization detector (PID) each sample horizon by inserting the probe in the “holes” left after sub-coring.
3.2 Results
The first week of DyeLIF characterization had daily production ranging from 86 m to 151 m for an average of 120 m/day (395 ft/day). A total of 25 locations were advanced to an average depth of 21.3 m (70 ft) bgs. Nine additional probes were advanced the following week while the soil coring and subsampling took place (two percussion soil sampling machines were available). The majority of the DyeLIF probing was done with a 5400 Geoprobe™ while a larger Geoprobe 7720 was used mostly for difficult pre-clearing duties (to 4.5 m bgs for utilities) and for the week 2 soil coring. The same tooling string performed without failure for the duration with just one internal mirror realignment necessary. The site’s DNAPL zone was fully bounded with the exception of one or two locations where infrastructure prohibited access to “move out” (and any DNAPL occurring at depths greater than 21.3m (70 ft) bgs, which was the maximum depth of the DyeLIF testing).
Week two’s soil sampling proved much more challenging. High core recovery was very difficult to achieve in the fine sands and silts occurring at this site, even with a crew that was experienced at this site and using the latest methods and tooling. Different coring methods, run lengths and operating variables were tried and eventually a sub-optimal but satisfactory approach was found. This involved use of the Geoprobe Macro-Core MC7™ sampler, which provides 75 mm (3-in) diameter cores in plastic tubes, with the larger size needed for the intensive subsampling. The MC7 tool was adapted to include a sealed piston above the soil core which was tied off in a fixed position while the core barrel was advanced through the target core interval (method adapted from Zapico et al., 1987). Run lengths of 0.91 m (3 ft) were used based on initial trials to maximize recovery. However, recovery still only ranged from about 50 to 85% with an average of 65%. A correction factor was applied to convert sub-sample positions in the core tubes to inferred “in-situ” depths.
Lateral heterogeneity also made encountering DyeLIF indicated DNAPL a “hit or miss proposition.” In a few cases immediately adjacent ‘duplicate’ DyeLIF pushes (<1 m apart) showed DNAPL at one location but not at similar depths in the adjacent location. Similarly in a few cases cores collected immediately adjacent to DyeLIF logs that indicated multiple DNAPL-impacts resulted in no DNAPL from the core subsampling at similar depth intervals. This is not unexpected given the complex and spatially variable DNAPL distributions expected at “aged” DNAPL sites (e.g. Parker et al., 2003). Persistence eventually yielded a sufficient number of cores that contained DNAPL affected soils and a total of 260 depth discrete horizons were sub-sampled from cores collected at five locations. Core depths were targeted to span the depth intervals where DNAPL was inferred from DyeLIF probing. All subsample depths were field tested with the PID, subjected to ORO visual DNAPL screening and DyeLIF testing, and over 50% (133) were subjected to quantitative VOC analyses. Figure 3 (top) illustrates one of the “raw” DyeLIF logs obtained in week one (DyeLIF 009). The light blue color of the upper 3m (10 ft) fluorescence is from loose sand that was placed in the hole after pre-clearing for utilities. Notice the unique waveform in callout one, showing the waveform from the sand. The yellow-filled “baseline” response is the weak fluorescence emitted by un-solvated dye particles injected into the formation. The other more intense blue-filled “spikes” at various depths are narrow responses of DNAPL. Notice the longer lifetimes and blue-shifted waveforms in callouts two, three, and four. This waveform shape is unique to DNAPL-solvated dye, establishing with high confidence the origin of the fluorescence response at those depths. The bottom callout shows the red-shifted waveform with very short lifetimes. The log is typical of logs in which modest DNAPL detections were made, but the pore saturation was low enough that the DNAPL response is obscured to some degree by un-solvated dye and other background fluorescence. Two other logs less than one meter away from DyeLIF 009 exhibited very large responses (150-200% Reference Emitter (RE) at 34 ft (10.3 m) bgs, while this log topped out at just 15% RE at that same depth). This high degree of lateral heterogeneity is not unusual when dealing with DNAPLs. For this reason the uphole comparisons (apples to apples) were also incorporated into the demonstration design, to account for the inevitably high variation between DyeLIF logs and co-located soil cores.
LIF waveforms are stored with each and every depth analyzed, allowing for post-processing using a non-negative least squares (NNLS) fitting process. The process uses a basis set of waveforms (nonsolvated dye, DNAPL-solvated dye, sand, etc.) to determine the origins of and then appropriate the fluorescence response. As discussed, there are dramatic spectral and temporal differences in the waveforms for DNAPL solvated dye vs. un-solvated dye. This allows us to mathematically “strip away” all but the DNAPL-specific responses, yielding high-confidence DNAPL-specific logs of DNAPL detections. Figure 3 (bottom) shows the same log after such post-processing. So that even small (but critical) DNAPL ganglia are readily recognized, the NNLS data is plotted in many columns, each five feet (1.5 m) in height. The fluorescence scaling of the processed log is held to 0-5 % RE so that even the lowest of pore saturations are obvious. The tiny response at 67.7 ft bgs is an example detection of a very small DNAPL ganglion. This post-processing requires about 5 minutes to conduct and was done on site immediately after the DyeLIF log was collected.
Notice also that in the raw DyeLIF log in Figure 3 (top) the back pressure and flow of injected dye solution are plotted vs. depth (two right-most data columns). The majority of logs show an increase in back pressure at or just below DNAPL responses, suggesting that the DNAPL distributed itself laterally to some degree when it encountered horizons of transition from higher to lower hydraulic conductivity. This was consistent with examination of soil cores showing DNAPL perched at interfaces transitioning to more silt content.
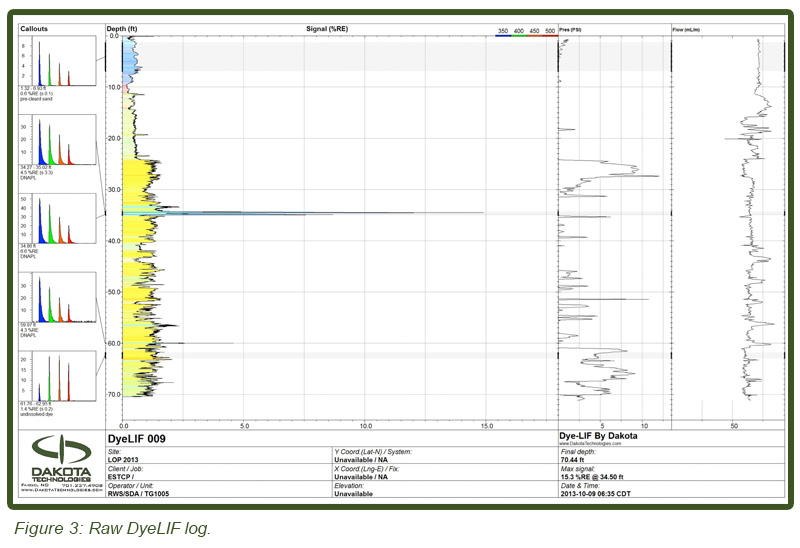
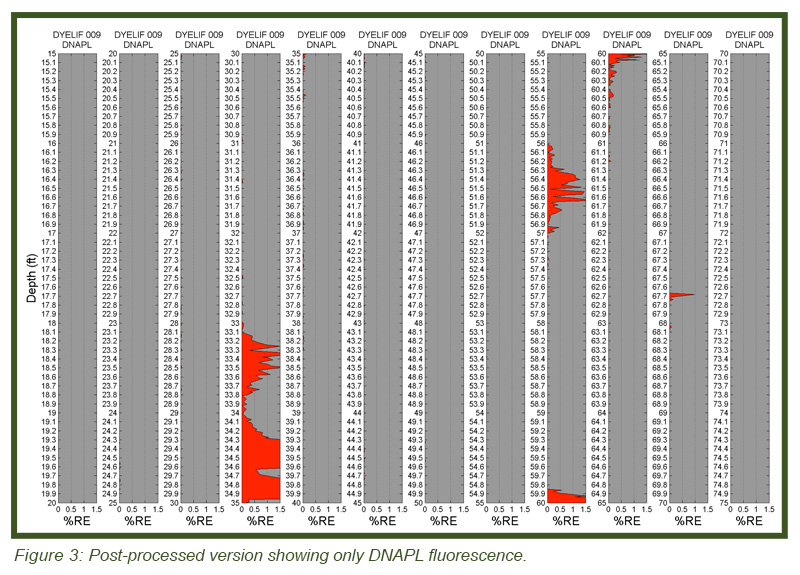
Figure 4 illustrates one example core containing DNAPL after high resolution subsampling. Notice that some of the ORO jars contain a “blood red” color, a positive indication of DNAPL presence with very abrupt transitions into and out of DNAPL. The ORO results were used as a guide in deciding which samples should be subjected to quantitative VOC analyses, from which DNAPL pore saturations were estimated using NAPLANAL software (Mariner et al., 1997; McCray and Cohen, 2003). Up-hole DyeLIF analysis of the 133 co-located soil sub-samples subjected to quantitative analyses showed excellent correlation with both the ORO screening and subsequent analytical lab results: 100% consistency between the three methods was achieved in samples found to contain > 2.5% DNAPL pore saturation and 98% consistency was found in soil with DNAPL pore saturations of > 0.7%. While the final results have yet to be assessed in detail, it appears that (for this site) the practical field limit of detection (LoD) for DyeLIF falls somewhere between 1.0% and 0.1 % DNAPL pore saturation. The PID screening results, while in general agreement that chlorinated solvent VOCs were present in the cores, showed little ability to discern DNAPL zones from the more diffusely distributed non-NAPL phase zones, due to very high responses and ‘pegging out’ in these high concentration zones.

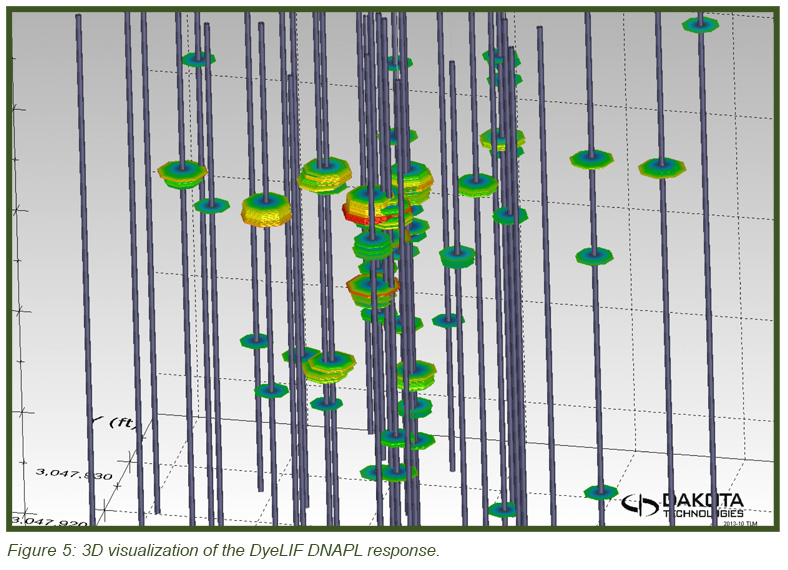
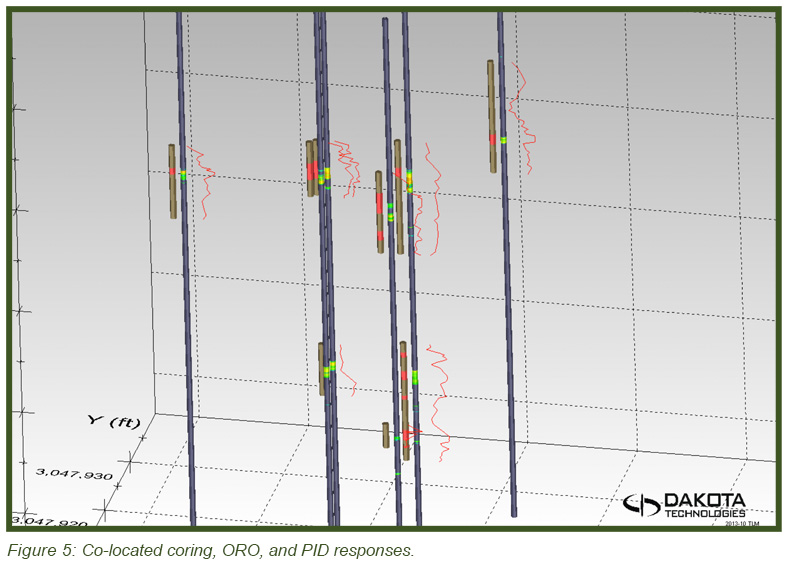
The image in Figure 5 (top) shows the heterogeneous distribution of the DNAPL in 3-dimensional space with the DyeLIF response represented as heat-diagram colorized disks attached to gray “posts” where the logs were conducted. The larger the disk and the “hotter” the color, the larger the DyeLIF response. The image at right contains the processed in-situ DyeLIF DNAPL-only logs from week one that were chosen for soil core co-sampling, alongside their confirmation core results. The tan-colored areas of the cores represent no ORO response and red indicates a positive ORO response while green, yellow, and red in the DyeLIF logs denotes positive responses (gray represents no DNAPL). Notice the red PID line plotted alongside that remains high along much of the sampling length, regardless of DNAPL presence or absence.
4. CONCLUSIONS
Chlorinated solvent sites, which formerly were not candidates for subsurface NAPL mapping using LIF, are now viable candidates for high-resolution LIF soil screening. The DyeLIF system’s ability to detect DNAPL is roughly equivalent to that of ORO soil screening of discrete samples, with the added benefit of very high production, higher data densities, automated electronic records of results, and no generation of waste. Also, this direct push technique provides in-situ characterization and is not subjected to uncertainty due to lower core recovery, fluids loss or redistribution in cores, etc. Plotting multiple DyeLIF logs in relation to their geospatial position is useful for visualizing the DNAPL body for engineering and remediation purposes allowing much more targeted remediation efforts. CPT advancement is planned for the next round of testing and the DyeLIF is scheduled for full commercialization in the spring of 2014.
ACKNOWLEDGEMENTS
Much of the work presented in this paper was funded by the Environmental Security Technology Certification Program (ESTCP) (Project No. ER-201121). Stone Environmental performed the field soil coring, some of the DyeLIF probing delivery and also the quantitative VOC analyses on the soil subsamples.
REFERENCES
Aldstadt, J., R. St. Germain, T. Grundl, R. Schweitzer, and S. Consulting. 2002. An in situ Laser-Induced Fluorescence System for Polycylic Aromatic Hydrocarbon-Contaminated Sediments.
Cohen, R.M., A.P. Bryda, S.T. Shaw, and C.P. Spalding. 1992. Evaluation of visual methods to detect NAPL in soil and water. Ground Water Monitoring Review, 12: 132–141.
Griffin, T.W., Watson, K.W. 2002. A comparison of field techniques for confirming dense nonaqueous phase liquids. Ground Water Monitoring and Remediation, 22: 48-59.
Mariner, P.E., M. Jin, and R.E. Jackson. 1997. An algorithm for the estimation of NAPL saturation and composition from typical soil chemical analyses. Ground Water Monitoring and Remediation 17(2): 122-129.
McAndrews, B., K. Heinze, and W. Diguiseppi. 2003. Defining TCE plume source areas using the membrane interface probe (MIP). Soil and Sediment Contamination 12(6): 799–813.
McCray, J.E., and R.M. Cohen. 2003. NAPLANAL: A tool for analyzing NAPL saturation and composition. Ground Water 41(3): 298-299.
Pankow, J.F., Cherry, J.A., 1996. Dense Chlorinated Solvents and other DNAPLs in Groundwater. Waterloo Press, Portland, OR.
Parker, B.L., Cherry, J.A., Chapman, S.W., Guilbeault, M.A., 2003. Review and analysis of chlorinated solvent dense nonaqueous phase liquid distributions in five sandy aquifers. Vadose Zone Journal, 2: 116–137.
USEPA. 2004. Site Characterization Technologies for DNAPL Investigations 165.
USEPA. 2005. Groundwater Sampling and Monitoring with DP Technologies 1-67.
Zapico, M.M., S. Vales, and J.A. Cherry. 1987. A wireline piston core barrel for sampling cohesionless sand and gravel below the water table. Ground Water Monitoring and Review, 7: 74–82.